OLED
Mechanism of light emission
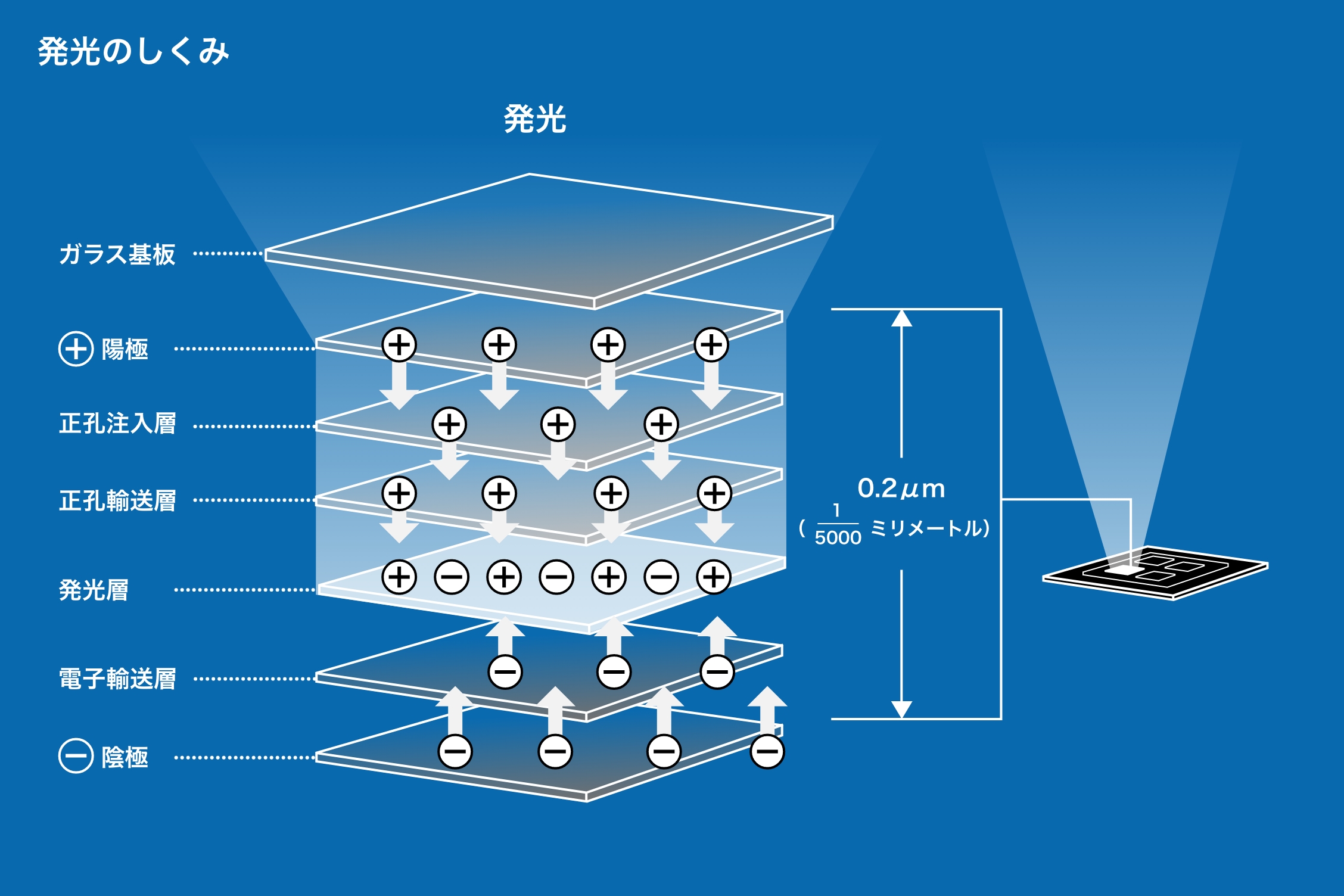
When an electric current is passed through certain organic compounds (light-emitting materials), the molecules are excited to a high-energy state by the electrical energy. When these molecules return to their original state, the difference in energy causes them to emit light, which is what makes OLED).
Organic compounds are in powder form and do not emit light as is. Light can be emitted by sandwiching a thin plate-shaped "light-emitting layer" of light-emitting material between layers that have functions such as controlling the flow of electrons. Materials designed in this way to emit light are called "elements."
Strengths of OLED displays
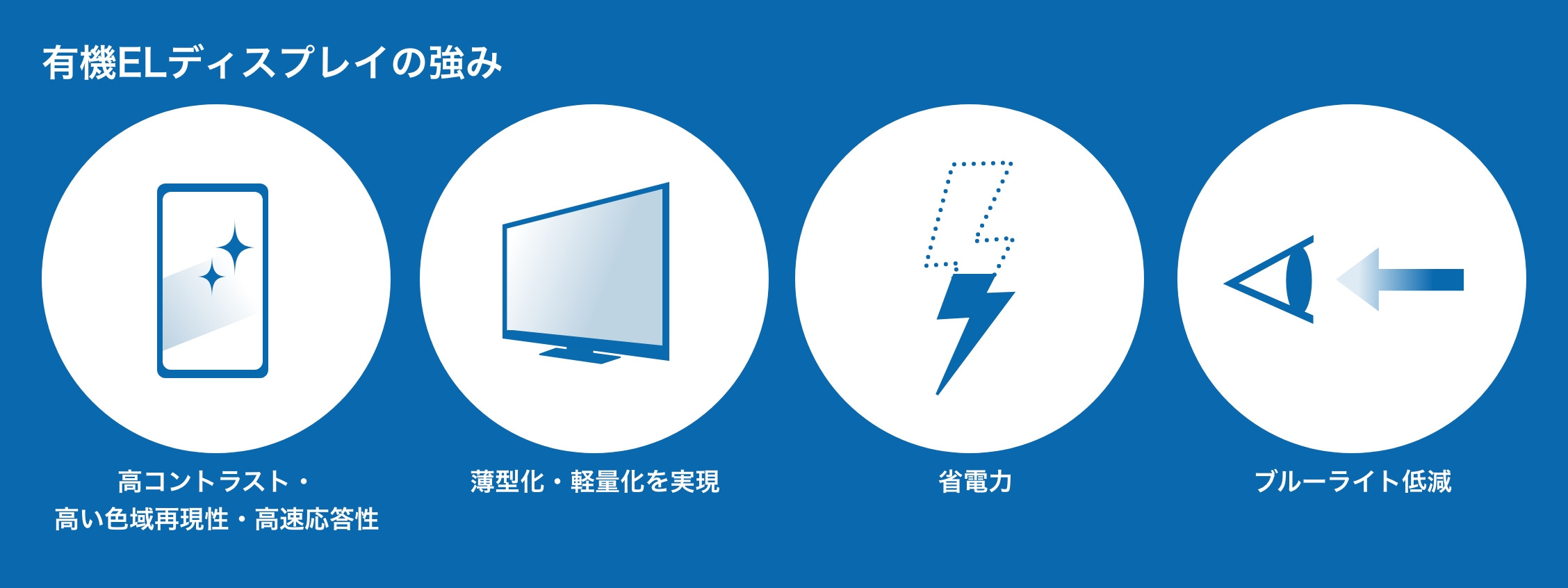
High contrast, high color gamut reproducibility, fast response
Since the elements themselves emit light, it is possible to express deep black and various colors found in nature. Additionally, the response time of the element is short, allowing for smooth video display.
Realized thinner and lighter weight
Unlike LCDs, which require a backlight, OLED can turn the light emitted by each pixel on and off, eliminating the need for a backlight. This makes it possible to make displays thinner and lighter, and allows for displays of a variety of shapes to be created.
power saving
OLED turn off light emission in areas that represent black, so they consume less power than LCDs, which have a constantly lit backlight.
blue light reduction
The blue emission wavelength can be freely adjusted using organic materials, making it possible to reduce harmful blue light.
Products using OLED materials
Due to the many advantages of OLED displays, they are increasingly being installed in a variety of electronic products, including high-performance devices such as smartphones and tablets, portable game consoles, and televisions.
It is also possible to realize flexible and transparent displays, and their applications are diverse. Smartphones and PCs equipped with foldable displays have also been released in recent years, and the market is expected to continue to grow in the future.
